Quick Links to More COVID-19 Information
On This Page
Symptoms
Signs and symptoms of COVID-19 are similar to influenza (flu) and include:
Cough

Headache
Shortness of breath or difficulty breathing

Sore throat

Fever or Chills
New loss of taste or smell

Fatigue
Congestion or runny nose
Muscle or body aches
Nausea or vomiting
Diarrhea
Symptoms may appear 2-14 days after exposure.
Most patients experience relatively mild symptoms and can recover at home. Some patients, like those with underlying medical conditions, may experience more severe respiratory illness. Learn more about COVID-19 symptoms.
Multisystem Inflammatory Syndrome in Children, MIS-C, is a serious but rare complication of COVID-19. It causes inflammation of certain body parts.
Prevent COVID-19
Core Prevention Strategies:
Stay up-to-date on immunizations.
Wash your hands often with soap and water for at least 20 seconds or use alcohol-based hand sanitizer.
Avoid touching your eyes, nose, and mouth with unwashed hands.

Cover your mouth and nose with a tissue when you cough or sneeze.
Clean frequently touched surfaces, like counters, handrails, and doorknobs.
Take steps for cleaner air.

Stay home if you are sick as much as possible. Avoid close contact with others.
Additional Prevention Strategies

Wear a face mask to protect yourself and others.
Avoid close contact with people who are sick.
Test for respiratory viruses if you feel sick to help you decide what to do next.
What to Do If You Are Sick
If you feel sick with COVID-like symptoms:

Stay home if you are sick as much as possible. Avoid close contact with others, including people you live with who are not sick.

Call your healthcare provider to discuss options.
If you have an emergency warning sign (including trouble breathing), get emergency medical care immediately.

Test for respiratory viruses to help you decide what to do next.
Is there a treatment for COVID-19?
Treatment is available for COVID-19 to help prevent severe disease. It works best if started early after symptoms start. Talk to a health care provider for more information.
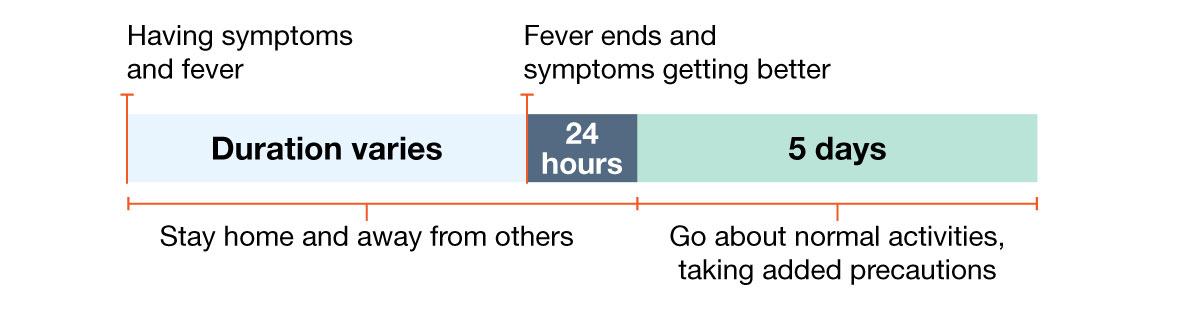
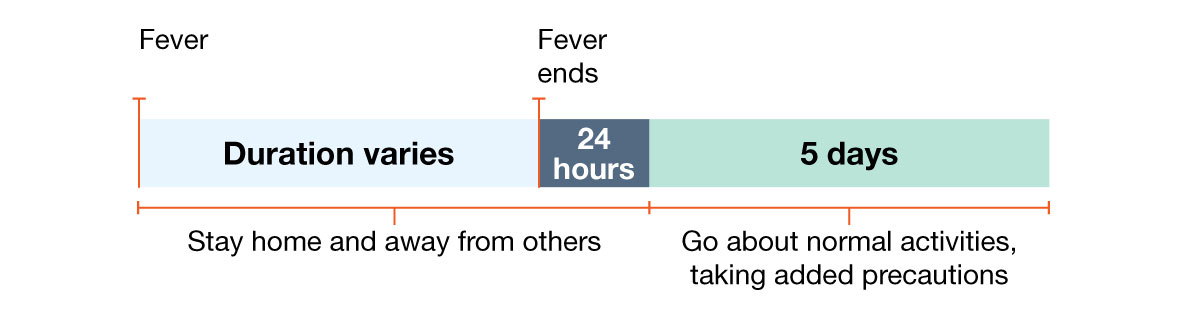
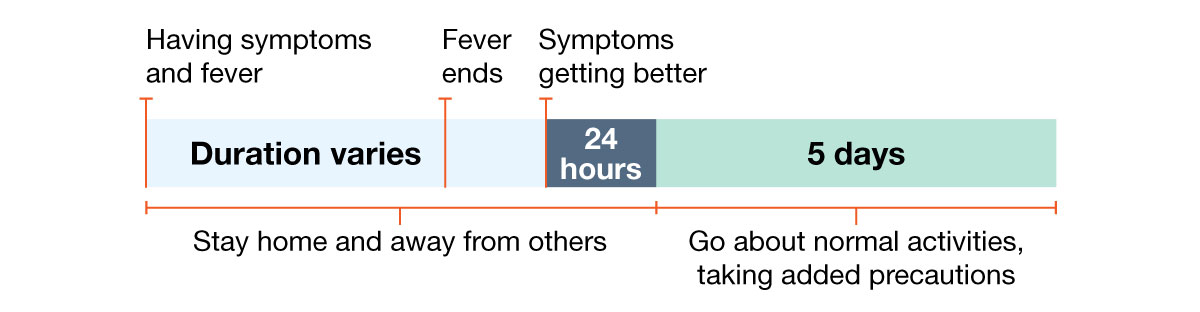
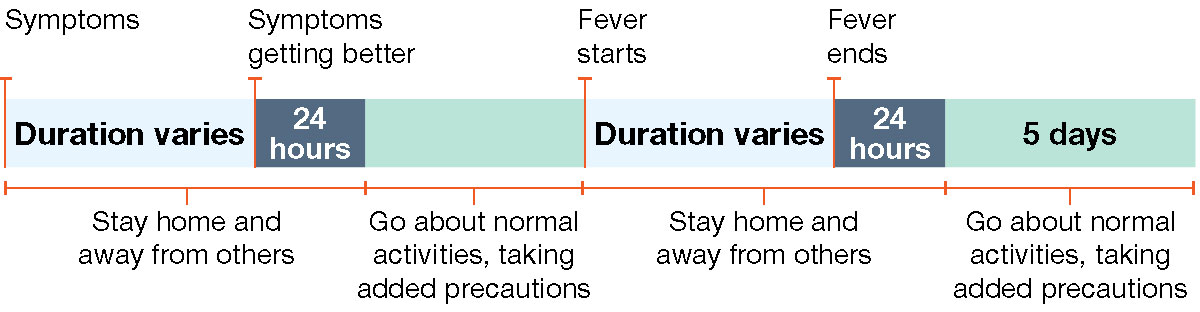
How long should I stay home and away from others?
Go back to your normal activities when BOTH of these are true:
- Your symptoms are getting better overall for at least 24 hours, AND
- You have no fever for at least 24 hours (without using fever-reducing medication).
Even when you feel better, you might still be able to spread the virus that made you sick. Once you go back to normal activities, take these added steps for the next 5 days when you will be around other people:

Wash your hands often with soap and water for at least 20 seconds or use alcohol-based hand sanitizer.

Clean frequently touched surfaces, like counters, handrails, and doorknobs.

Take steps for cleaner air if you have to be around other people.

Wear a face mask around others.

Put extra space between yourself and other people.

Test for respiratory viruses if you have to be around others, especially people at risk for severe illness.
Remember, if you develop a fever or start to feel worse after you go back to normal activities, stay home and away from other people until you feel better and are fever free for 24 hours again.
If you are sick and live or work in a health care setting, there is different guidance for you to follow.
Examples:
Example 1: Person with fever and symptoms.
Example 2: Person with fever but no other symptoms.
Example 3: Person with fever and other symptoms. Fever ends but other symptoms take longer to improve.
Example 4: Person gets better and then gets a fever again.
COVID-19 Vaccination
For vaccines to be broadly distributed and administered, they must go through a federal review process. The standard process begins with U.S. Food and Drug Administration (FDA) approval, followed by review and recommendation by the Advisory Committee on Immunization Practices (ACIP), and finalized with sign off by the U.S. Department of Health and Human Services. As the federal approval process for the 2025-2026 COVID-19 vaccine recommendations is actively underway and the state considers additional action, information is subject to change and updates will be made available as information is confirmed.
The FDA approved the 2025-2026 COVID-19 vaccine for certain populations on August 27, 2025. The federal Advisory Committee on Immunization Practices (ACIP) is scheduled to meet September 18-19 to review the FDA approval and determine recommendations for the 2025-2026 COVID-19 vaccine use. These recommendations will then require final sign-off from the U.S. Department of Health and Human Services before vaccines become more broadly available.
The Maine Center for Disease Control and Prevention (Maine CDC) strongly values the personal and public health benefits of immunization and remains committed to providing clear, evidence-based information that allows the people of Maine to make informed health care decisions. The Maine CDC is assessing changes by the FDA to COVID-19 vaccine eligibility, in advance of further guidance from ACIP expected later this month. The goal of the Administration, including the Maine CDC, will be to prevent any medically-unnecessary restriction of vaccines, so that Maine people may be able to consult with their health care provider and decide what is in the best interest of their health.
To provide clarity to the extent possible while federal action is pending, the Maine CDC is sharing the following information, which is current as of September 9, 2025.
Frequently Asked Questions
Who is eligible for a COVID-19 vaccine?
The U.S. Food and Drug Administration (FDA) has approved the 2025-2026 COVID-19 vaccine for individuals 65 years and older and people 6 months to 64 years old with conditions that put them at high risk for severe illness from COVID. The U.S. CDC lists a wide range of conditions and behaviors that are considered higher risk for COVID-19, including pregnancy or recent pregnancy.
Individuals who do not meet these criteria but would like to get the COVID-19 vaccine may be able to do so by getting a prescription from your doctor or another medical provider.
How can I get a COVID-19 vaccine today?
Until ACIP makes its recommendations and they are signed off by the U.S. Department of Health and Human Services, the only way to get the 2025-2026 COVID-19 vaccine is through your health care provider or with a prescription. If you would like a COVID-19 vaccine today, contact your provider to see if they have vaccine. If they do not, ask your provider for a prescription that you can get filled (administered) at a participating pharmacy. Please contact the pharmacy ahead of time to confirm that they are administering the vaccine.
Please note: many providers order vaccines for children 6 months to 18 years through the Maine Immunization Program. The 2025-2026 COVID-19 vaccine is not yet available for ordering through this program and will not be until there is additional guidance following the upcoming ACIP meeting scheduled in September. This may limit what providers have available for children 6 months to 18 years old.
Why can’t I get a COVID-19 vaccine at my local pharmacy like I have in the past?
Under Maine law, pharmacists are limited in administering the vaccine without a prescription until ACIP makes recommendations related to the 2025-2026 COVID-19 vaccine. This is different from previous years due to the delay in ACIP recommendations; the Maine CDC, pharmacies, and others are considering steps that can be taken to improve access to the vaccine given these Federal changes.
Once I have a prescription, where can I get a COVID-19 vaccine?
You may be able to use a prescription from your provider to get a vaccine at your local pharmacy, but you should call ahead to make sure they have it in stock and are choosing to administer. Your health care provider may also have the 2025-2026 COVID-19 vaccine available in their office.
Will my insurance cover the COVID-19 vaccine?
You will need to check with your health insurance plan to understand whether the 2025-2026 COVID-19 vaccine is covered. Unlike during the pandemic, not all insurance carriers cover the COVID-19 vaccine, and there are fewer programs to cover the cost for individuals who are uninsured or under-insured.
MaineCare, Maine’s Medicaid program, covers the COVID-19 vaccine for members who are within the FDA approved population or have a prescription from a medical provider.
Has the COVID-19 vaccine changed for this year? Why was the 2025-2026 vaccine approved for fewer people?
The 2025-2026 COVID-19 vaccine is updated based on currently-circulating strains of the COVID-19 virus. While the FDA approved the vaccine for a smaller segment of the population than in the past, its approval indicates the vaccine is considered safe for an otherwise healthy person to get the vaccine if they choose.
Long COVID Resources
What resources can I find online about Long COVID?
Where should I go for clinical care? Should I go to a Long COVID specialist?
- If you experience Long COVID symptoms, contact your primary care provider. They will help you create a plan for care. If you have ongoing symptoms that need special testing, your primary care provider might refer you to a specialist. They may refer you to a Post COVID Care Center if your symptoms need extra management.
Resources
COVID‑19 Factsheet (PDF): عربي | Français (PDF) | Kreyòl Ayisyen (PDF) | ខ្មែរ (PDF) | Lingala (PDF) | Português (PDF) | Soomaali (PDF) | Español (PDF) | Kiswahili (PDF) | Tiếng Việt (PDF)
State of Maine COVID-19 Website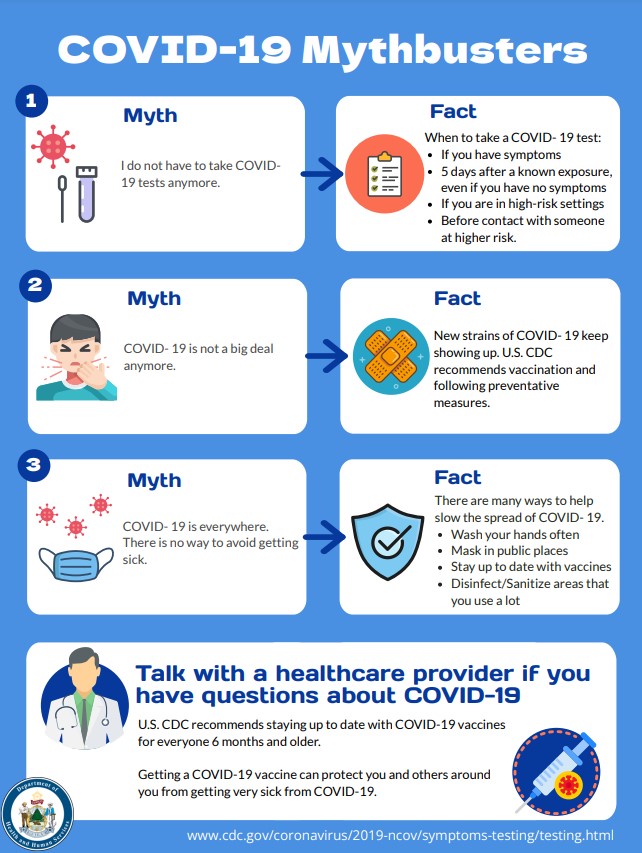
COVID Mythbusters (PDF)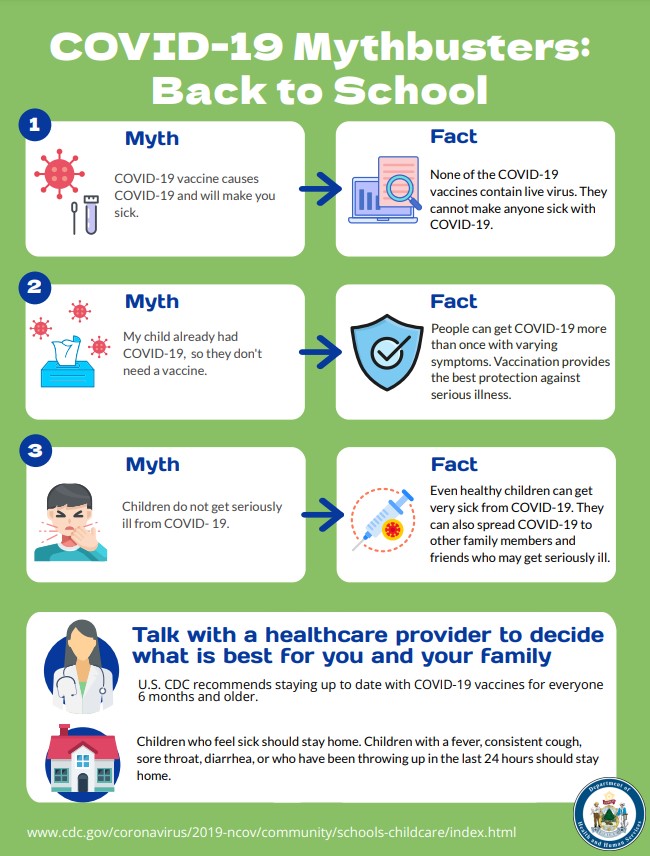
COVID Mythbusters - Back to School (PDF)
COVID Mythbusters - Pregnancy (PDF)
COVID Mythbusters - Vaccine (PDF)
Symptoms of Coronavirus (COVID-19) (PDF)
Multilingual Wash Your Hands Poster (PDF)
Wash Your Hands Poster (PDF)
Wash Hands Reminder (Color) (PDF)
How to Self Collect an Anterior Nasal Swab for COVID-19 Testing (PDF)
US CDC Types of Masks and Respirators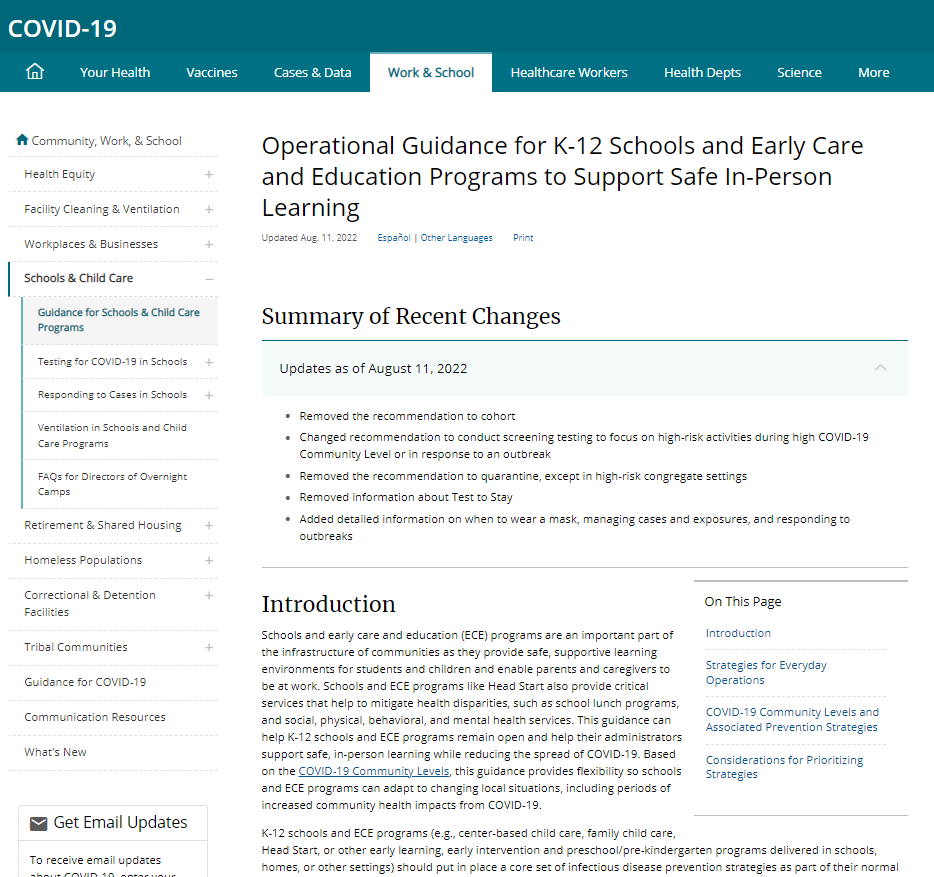
U.S. CDC Operational Guidance for K-12 Schools and Early Care and Education Programs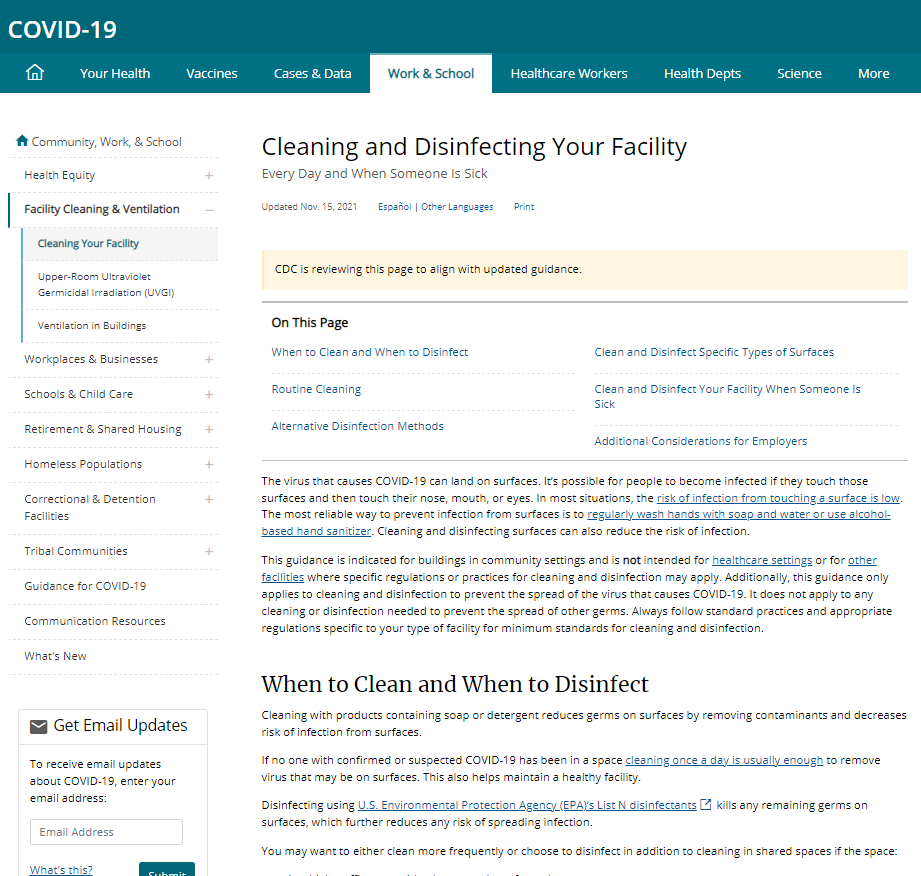
U.S. CDC Cleaning and Disinfection Recommendations for Facilities